About
Introduction
Education and career
Publications
- van Gulijk M, Dammeijer F, Aerts JGJV, Vroman H (2018). Combination Strategies to Optimize Efficacy of Dendritic Cell-Based Immunotherapy.
Front Immunol. 2018 Dec 5;9:2759. - Aerts JGJV, de Goeje PL, Cornelissen R, Kaijen-Lambers MEH, Bezemer K, van der Leest CH, Mahaweni NM, Kunert A, Eskens FALM, Waasdorp C, Braakman E, van der Holt B, Vulto AG, Hendriks RW, Hegmans JPJJ, Hoogsteden HC (2018). Autologous Dendritic Cells Pulsed with Allogeneic Tumor Cell Lysate in Mesothelioma: From Mouse to Human.
Clin Cancer Res. 15;24(4):766-776. - Dammeijer F, Lievense LA, Kaijen-Lambers ME, van Nimwegen M, Bezemer K, Hegmans JP, van Hall T, Aerts JG (2017). Depletion of Tumor-Associated Macrophages with a CSF-1R Kinase Inhibitor Enhances Antitumor Immunity and Survival Induced by DC Immunotherapy.
Cancer Immunol Res. 5(7):535-546. - De Goeje PL, Smit EF, Waasdorp C, Schram MTB, Kaijen-Lambers MEH, Bezemer K, de Mol M, Hartemink KJ, Nuyttens JJME, Maat APWM, Hegmans JPJJ, Hendriks RW, Senan S, Aerts JGJV (2017). Stereotactic Ablative Radiotherapy Induces Peripheral T-Cell Activation in Patients with Early-Stage Lung Cancer.
Am J Respir Crit Care Med. 1;196(9):1224-1227. - Dammeijer F, Lievense LA, Veerman GD, Hoogsteden HC, Hegmans JP, Arends LR, Aerts JG (2016). Efficacy of Tumor Vaccines and Cellular Immunotherapies in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis.
J Clin Oncol. 10;34(26):3204-12.
More
Based on previous results in animal models, three phase I/II clinical trials were performed involving mesothelioma patients, who were treated with five DC vaccinations. These trials have shown that DC therapy is safe and feasible in mesothelioma patients, and more importantly, several patients showed a reduction in tumor load, indicating efficacy of DC vaccination. This research is now continued with a phase III clinical trial in which efficacy will be compared with best standard care. DC therapy can be hampered by the strong immunosuppressive environment of the tumor, as the tumor microenvironment comprises various immune suppressive cell types, such as regulatory T cells, myeloid derived suppressor cells and tumor-associated macrophages. Therefore, in our murine mesothelioma model we are optimizing DC therapy through combination with other immunomodulatory agents that target the immunosuppressive environment of the tumor.
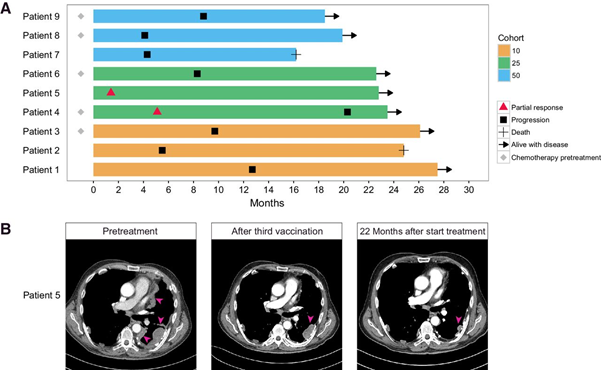
Figure: Overall survival and RECIST responses after first vaccination.
A. Swimmer plot of patients in phase I clinical trial with MesoPher treatment. Overall survival of patients since date of first vaccination is represented by the filled bars. Start and end of RECIST responses are depicted by the red triangles and black squares, respectively. First evaluation of response was after 6 weeks for all patients. Patients treated with chemotherapy prior to inclusion in the study are depicted with a gray diamond. The other patients were treatment naïve. B. Pre-and postvaccination CT scans of patient 5. CT scans with contrast of the ongoing response of one treatment-naïve patient receiving allogeneic lysate pulsed autologous DC vaccination. Tumor mass is indicated with pink arrows. All lesions were solid. This patient was treatment naïve and received 5 doses of 25 million DCs. Pretreatment tumor burden decreased with 70% after third vaccination (6 weeks after start of treatment) and continued to decrease (from: Aerts JGJV, et al, Clin Cancer Res. 2018).