What we do
About our project
Functional imaging of biological processes in pre-clinical cancer models
Custom-made genetically engineered pre-clinical mouse models and orthotopic tumor mouse models are developed to test treatments that are rationally designed based on molecular mechanistic insights from the ‘Mechanisms of the DNA damage response’ research line. This involves unique Erasmus MC resources for 3D imaging to identify and locate molecular processes in action in vivo in live mice in a longitudinal manner. The ultimate goal, in combination with the ex vivo functional assays on patient-derived materials described below, should be to develop (biology-based) image-guided, adaptive and targeted cancer therapies.
The DNA damage response as a functional tool in precision cancer therapy
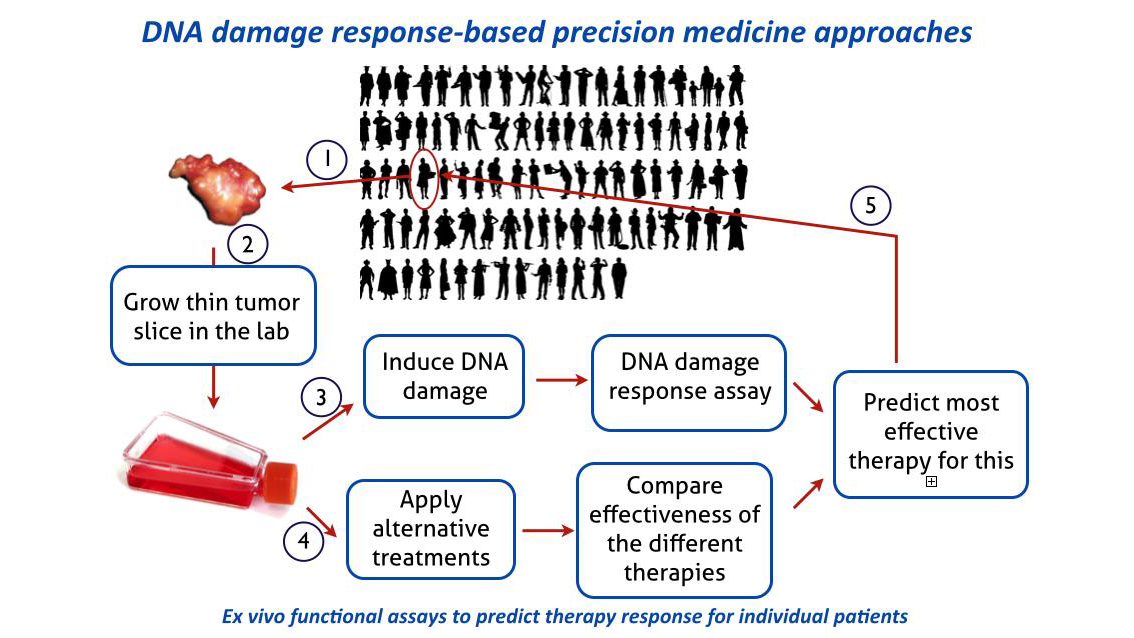
Based on extensive analysis of the DNA damage response in cell culture the Department has developed a clinic-to-lab pipeline for breast cancer patients that allows testing, in a functional ex vivo assay, aspects of the DNA damage response in viable (organotypic) slices of tumor material from individual patients. In the case of breast cancer this assays allows selection of patients for a specific targeted therapy. This method is now poised to move from the lab back to the clinic through a clinical trial in collaboration with the Erasmus MC Department of Medical Oncology. The responses observed in the ex vivo DNA damage response on biopsies from individual breast cancer patients will be correlated with outcome of their clinical treatment.
Our research focus
Relevance for individual patients
A major goal within this research line should be to obtain a direct functional appraisal of the DNA damage response capacity for individual patient tumors. Based on this principle, functional assays will be developed for multiple DNA damage response reactions and extend beyond breast cancer to other tumor sites. These assays will ultimately identify specific defects in DNA damage response that can be exploited in the exciting new strategy of synthetic lethality approaches, as pioneered for hereditary breast and ovarian cancer (see video).
Systematic exploration
The potential for synthetic lethality is in principle general and needs to be systematically explored in general for other tumors or for other DNA damage response defects and in the context of mechanism-based chronotherapeutic cancer treatment protocols. Connecting this research line to the Human Organ and Disease Model Technology (hDMT) Institute provides opportunities to adopt the functional DNA damage response assays on tumors to a microfluidic chip format for higher throughput greatly enhancing the utility in a clinical setting. By focusing research around precision therapies the Department will easily connect to the ‘Nationale Wetenschaps Agenda’ exemplar route ‘Personalized Medicine’.
Proton therapy
Furthermore, our team is initiating research on the DNA damage response towards protons through the close and long-standing collaboration with the Department of Radiation Oncology and with a collaboration to be initiated with Holland PTC, in the context of Medical Delta. Understanding the molecular mechanism and cellular pathways of the DNA damage response in reaction to protons will provide knowledge not only for enlarging the therapeutic window of proton therapy, but also for designing synergizing combination therapies.

