What we do
About our project
Background
Mental illness is the largest cause of disability in Europe, accounting for 22% of the total health burden. Half of adult mental illnesses develop before age 14, making early life an important window for prevention and intervention. Behavioral problems such as inattention and hyperactivity are common reasons for child treatment referrals and early predictors of poor adult mental health. In light of increasing rates of behavioral and neurodevelopmental problems in children, the EU has called for urgent action to identify developmental pathways leading to mental health issues to improve prevention, detection, and treatment strategies.
Epigenetic timing effects on mental health
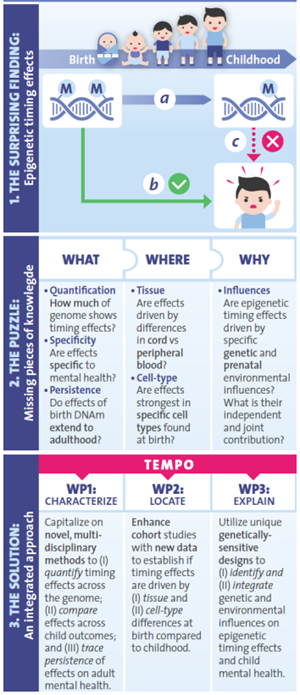
Recently it has been observed that common mental health problems in children are associated with epigenetic patterns at birth (Figure 1b) – a mechanism that regulates gene activity in response to genetic and environmental influences. Curiously, however, this association is ‘lost’ when measuring the same epigenetic patterns later in development (Figure 1c). This discovery is highly unexpected and potentially ground-breaking: the existence of timing effects could lead to new insights into the origins of mental health and open much-needed opportunities for early risk detection. Yet, what factors drive these timing effects, how they manifest and why they occur is currently unknown.
The TEMPO project
TEMPO combines innovative, multidisciplinary approaches and the generation of new data in longitudinal European cohorts to:
(i) Systematically characterize unknown properties of epigenetic timing effects, including their genomic scale, specificity to mental health and persistence into adulthood, using advanced quantitative methods;
(ii) Locate epigenetic timing effects with greater precision, by establishing whether effects are driven by epigenetic patterns in specific tissues and cell-types that are found at birth but not later in life;
(iii) Explain timing effects, by disentangling their genetic and environmental origins, drawing on the power of genetically sensitive designs.
Implications
As well as leading a breakthrough in the field of psychiatric epigenetics and addressing a major knowledge gap at the intersection of biological and psychological sciences, TEMPO has the potential to set in motion a paradigm shift in the way that we conceptualize, understand and approach child mental health.
Our research focus
Setting the scene for TEMPO
In the first year of TEMPO, we focused on establishing the team and setting up procedures for data access. TEMPO's rationale and priorities have been disseminated through expert commentaries and opinion pieces reflecting on progress, challenges and current research priorities within the field of psychiatric epigenetics. Additionally we advocated the need for developmental and interdisciplinary research within the emerging field of neuroimaging epigenetics, in a systematic review on the topic, as well as establishing a new collaborative initiative - the Methylation, Imaging, and NeuroDevelopment (MIND) Consortium – to address these unmet needs and accelerate our understanding of DNA methylation-brain dynamics.
Aim 1. WHAT: Characterizing epigenetic timing effects on child mental health
We aim to characterize key unknown properties of epigenetic timing effects, by using advanced statistical approaches on data from large-scale longitudinal birth cohorts. So far, our work – pooling together data from 33 cohorts, involving over 60 international collaborators – confirms the existence of epigenetic timing effects. We find that DNAm at birth is indeed a more significant predictor of several child outcomes, compared to when DNAm is measured later in development. Importantly, we find that epigenetic signals are highly temporally-specific: what we see at one time point may thus be missed if we measure epigenetic markers at a different time point.
Aim 2. WHERE: Locating epigenetic timing effects with greater precision
DNAm patterns differ between cell-types. This raises the possibility that the epigenetic timing effects we observe reflect the use of different tissues, and intriguingly, that specific cell-types found specifically in cord blood at birth may show greater potential than others in predicting mental health outcomes. To address this, we are generating new data that will enable us to establish the role of (i) tissue, by compare cord blood with neonatal peripheral blood from heel pricks, and (ii) cell-type, by comparing the predictive power of cell-types that are either specific to cord blood or present across development.
Aim 3. WHY: Identifying the genetic and environmental origins of epigenetic timing effects
Our third objective is to develop an explanatory model of epigenetic timing effects on mental health. Here, we want to understand why these timing effects occur and what factors lie behind the DNAm signals detected at birth. A key question we want to address is the extent to which these early signals capture prenatal environmental influences and/or genetic liabilities for mental health problems. To this end, we are drawing on powerful, genetically informed methods to identify and integrate these influences. Findings have the potential to elucidate etiological pathways and pinpoint new targets for prevention.

Funds & Grants
Collaborations
Collaborations outside of Erasmus MC
Publications
- Epigenetics and ADHD: Reflections on current knowledge, research priorities and translational potential. Cecil CAM†, Nigg JT. (2022) Molecular Diagnosis & Therapy.
- Epigenetics applied to child and adolescent mental health: Progress, challenges and opportunities. Cecil CAM†, Neumann A, Walton E. (2022) JCPP Advances.
- A systematic review of neuroimaging epigenetic research: Calling for an increased focus on development. Walton E, Baltramonaityte V, Calhoun V, Heijmans B, Thompson P, Cecil CAM. (2023) Molecular Psychiatry.
- Mapping gene by early life stress interactions on child subcortical brain structures: A genome-wide prospective study. Bolhuis K*, Mulder RH*, de Mol CL, Defina S, Warrier V, White T, Tiemeier H, Muetzel RL, Cecil CAM†. (2022) JCPP Advances.
- DNA methylation and general psychopathology in childhood: An epigenome-wide meta-analysis from the PACE consortium. Rijlaarsdam J*, Cosin C*, Schellhas L*, Abrishamcar S, Malmberg A, Caramaschi D**, Alemany S**, Cecil CAM**. (2022)
- DNA Methylation at Birth and Fine Motor Ability in Childhood: An Epigenome-wide Association Study with Replication. Serdarevic F, Luo M, Karabegović I, Binter A, Alemany S, Mutzel R, Guxens M, Bustamante M, Hajdarpasic A, White T, Felix JF, Cecil CAM, Tiemeier H. (In Press) Epigenetics.
- DNA Methylation at Birth and Lateral Ventricular Volume in Childhood: A Neuroimaging Epigenetics Study. Luo M, Walton E, Neumann A, Thio CHL, Felix JF, van Ijzendoorn MH, Pappa I, Cecil CAM. (In Press) Journal of Child Psychology and Psychiatry.
- Gestational epigenetic age and ADHD symptoms in childhood: A prospective, multi-cohort study. Salontaji K, Hafton KL, Sanders F, Page CM, Walton E, Felix JF, Bekkus M, Bohlin J, Tiemeier H, Cecil CAM. (2024) Molecular Psychiatry.
- Epigenetic timing effects on child developmental outcomes: A longitudinal meta-regression of findings from the Pregnancy And Childhood Epigenetics Consortium. Neumann A, Sammallahti S, Cosin-Tomas M, Reese SE, Suderman M, Alemany S, Cecil C. (2024) medRxiv.
Our team
Principal investigator
- Dr. Charlotte Cecil, PhD
Associate Professor
Team members
- Dr. Alexander Neumann, PhD
Postdoctoral researcher - Dr. Isabel Schuurmans, PhD
Postdoctoral researcher - Mahnoor Sulaiman, MSc
PhD Candidate