About Prof. dr. R.J. (Robbert) Rottier
Introduction
The lung development group is part of the department of Pediatric Surgery of the Sophia Children’s Hospital, a NFU recognized Center of Excellence for pediatric surgical index diagnoses (Dept. Head Prof Rene Wijnen). The Sophia Children’s Hospital is a referral center for major congenital anomalies, among which are foregut anomalies, like congenital diaphragmatic hernia (CDH), pulmonary hypertension of the newborn (PHN), Alveolar Capillary Dysplasia (ACD) and esophageal atresia with tracheoesophageal fistulas (EA/TOF).
In addition, increasing interest has extended towards the Pediatric lung disease, Bronchopulmonary Dysplasia (BPD), which is an injury of the lung due to (extreme) prematurity. The Principal Investigator of the lung development group is Robbert Rottier, and was initiated in the 90’s of the last century by Prof Dick Tibboel. The laboratory is embedded within the department of Cell Biology and is an integral part of three Academic Centers of Excellence (ACE): Systems Biomedicine, Anatomical Congenital Malformations, and Pulmonary Hypertension. The overall aim of the research is to improve our understanding of foregut abnormalities by studying lung development in health and disease.

Legend: Dr Rottier’s research group, December 2019
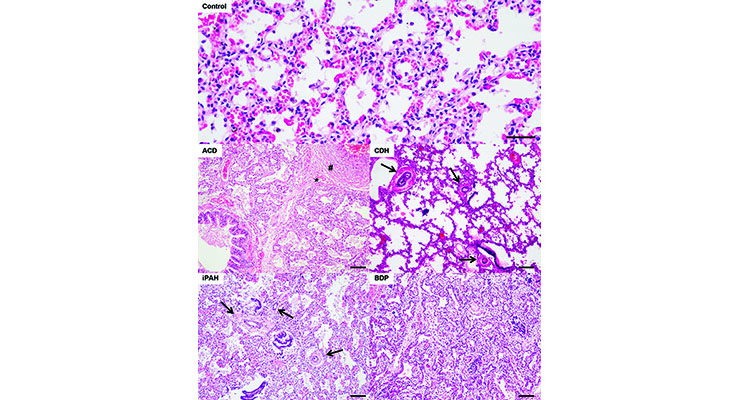
Characteristic histology of four pulmonary vascular disease samples.
Hematoxylin and eosin staining of human lungs: control, idiopathic pulmonary hypertension (iPAH), congenital diaphragmatic hernia (CDH), alveolar capillary dysplasia (ACD) and bronchopulmonary dysplasia (BPD). Scale bars 100µm. iPAH: thickening of the arteries (arrows), CDH: excessive muscularisation of the arteries (arrows), ACD: medial hypertrophy and muscularisation (#), malpositioning of the pulmonary veins (*) and central positioning of the capillaries in the alveolar septa, BPD: fibrosis with widening of the alveolar septa. (Kool et al, 2014)
Within the scope of this general aim, several lines of research are currently pursued, which partly overlap:
1: How does the airway epithelium differentiate and regenerate?
This links early developmental defects with adult chronic lung disorders, which is increasingly recognized as an important issue.
2: What is the origin of the pulmonary vascular changes and are there possible overlapping pathways between different pulmonary vascular diseases?
3: What is the interaction between diaphragm muscle cells and the supportive cells?
CDH is characterized by structural abnormalities of the diaphragm, which results in the presence of abdominal organs in the thoracic cavity. This may provide insights in the relation between the diaphragm defect and the lung abnormalities observed in CDH patients.
4:What is the role of lung mesenchymal stem cells and the lung microbiome in modulating lung development?
5: What do airways of BPD patients and COPD patients have in common?
This relates to the observations that early events in life that cause pediatric lung disease, increases the susceptibility to develop chronic lung disease later in life. This project is in collaboration with prof Hiemstra (LUMC) and Dr Hylkema (UMCG).
Our research is also related to the notion that early events leading to pre – and perinatal lung diseases, result in lungs that are susceptible to develop chronic lung diseases later in life. Importantly, chronic lung disorders, such as chronic obstructive pulmonary disease (COPD) and asthma, affect an estimated one million patients in the Netherlands. Strikingly, all lung-associated diseases combined, including respiratory infections and lung cancer, surpass cardiovascular disease as the main cause of death according to WHO health statistics.
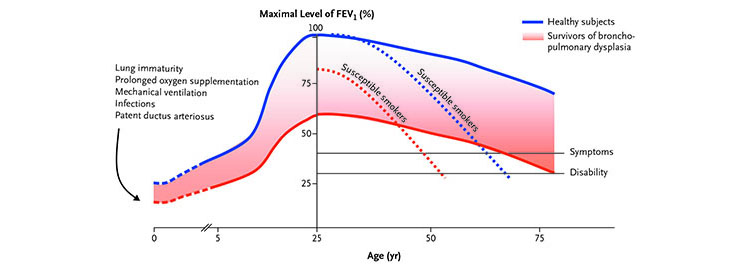
Theoretical Model of Changes in FEV1 in Survivors of Bronchopulmonary Dysplasia and Healthy Subjects According to Age. (Baraldi and Filippone, 2007)
Links to relevant websites:
Research projects
Introduction
The adult lung is a complex, highly organized organ and indispensable for life. It can be functionally separated into the bronchial system, required for the transport, filtering, heating and moistening of the inhaled air, and the respiratory system, required for the actual gas exchange of the inhaled oxygen and the carbon dioxide rich blood. The lung occupies the thoracic cavity and originates as a derivative of the primitive gut, which arises when endodermal cells start to fold into a tubular structure after gastrulation. The rostral part, the foregut, gives rise to a number of structures, like the thyroid, lung and liver through a process called budding. Lung development can be divided into five separate, but in time overlapping phases (Mo = Mouse; Hu = Human):
- embryonic (Mo E9–11.5; Hu Wk 3–7),
- pseudoglandular (Mo E11.5–16.5; Hu Wk 5–17),
- canalicular (Mo E16.5–17.5; Hu Wk 16–26),
- saccular (Mo E17.5–PN5; Hu Wk 26–36),
- alveolar (Mo PN5–30; Hu Wk 36–3 years).

Early stages of lung development (Maeda et al, 2007)
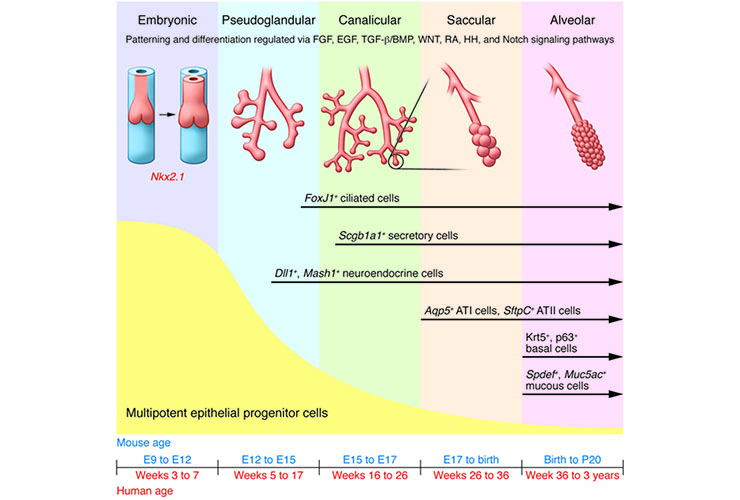
Lung development in both mouse and human progresses through five overlapping phases based on successive branching: embryonic, pseudoglandular, canalicular, saccular, and alveolar (9). The epithelium is initially composed of multipotent progenitor cells that proliferate and differentiate through development to yield more restricted, differentiated progeny that make up the developed lung epithelium. Signaling pathways that maintain the multipotent progenitor pool are indicated. HH, hedgehog; RA, retinoic acid (Rackley and Stripp, JCI 2012)
During these phases, the primitive lung bud, which extrudes from the foregut into the surrounding mesenchyme, starts to develop into the bronchial tree. The adjacent mesenchyme secretes growth factors to induce the primitive lung airway to grow and branch. The repetitive branching and growth of the developing airway, a process generally called branching morphogenesis, ultimately leads to the highly ordered bronchial tree of the lung. In later phases, the alveoli, the gas exchange units, mature and differentiate, in part postnatally.
As a consequence, lung abnormalities that occur during development or early in life, such as CDH, BPD and PHN, result in life threatening diseases, which require intensive medical interventions, both surgical and non-surgical. Understanding the processes and molecular pathways that are fundamental in the origin of congenital and acquired lung abnormalities early in life is essential to provide improved treatment options to these patients in the future. Moreover, it will generate insights in the origin and progression of chronic lung diseases.
Epithelial differentiation
Premature born infants face immediate problems to adapt to extra-uterine life. Their adaptation to oxygen requires many pulmonary adjustments, not only in the vascular system (see vascular development), but also in the epithelium of the airways. The treatment modalities at the intensive care unit to rescue these newborns also induce damage in the lungs, which needs to be repaired. It is thought that the molecular processes regulating pulmonary development are re-initiated during repair of the damaged lung. We are interested in understanding factors that are involved in epithelial differentiation, and the cells that are involved in differentiation and repair of the airways. Our focus has been on several transcription factors, like Sox2, Hif2α and Hif3α (examples: Gontan et al., Dev Biol 317, 296-309: 2008; Huang et al., Am J Respir Cell Mol Biol 46, 224-232: 2012; Ochieng et al., Am J Respir Cell Mol Biol 51, 311-322: 2014).
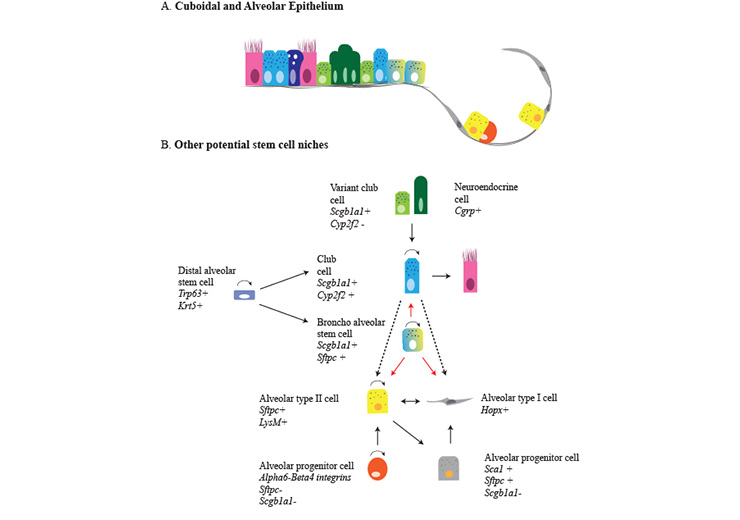
Regeneration of distal and alveolar airway epithelium after injury. a The small airways lack basal cells and consist of cuboidal epithelium, containing secretory and ciliated cells, as well as clusters of neuroendocrine cells. The cuboidal epithelium passes into a broncho-alveolar duct junction which is the niche of broncho-alveolar stem cells. The alveolar epithelium consists of alveolar type I, type II cells and alveolar progenitor cells. b Variant club cells (Cyp2f2−) are a variant of secretory cells that survive naphtalene injury and give rise to cyp2f2+ club cells. Lineage tracing of Cgrp+ cells showed that after depletion of club cells by naphtalene injury neuroendocrine cells contribute to the regeneration of these cells. At the broncho-alveolar duct junction, broncho-alveolar stem cells were isolated and shown to differentiate into bronchiolar and alveolar lineages in culture (dashed lines). Scgb1a1+ cells have the potential to form alveolar type I and type II cells after bleomycin injury, but not after hyperoxia-induced injury (dashed line). AT-II cells can self-renew and differentiate to AT-I cells. After pneumonectomy, a contribution of AT-I cells to regenerate AT-II cells was observed. An alveolar progenitor cell expressing α6-β4 integrins can regenerate AT-II cells after injury. Yet another cell type was identified expressing Sca1+ arising from AT-II cells and regenerating AT-I cells. Distal alveolar stem cells appear after severe injury and give rise to secretory and alveolar cells (Schilders et al, 2016)
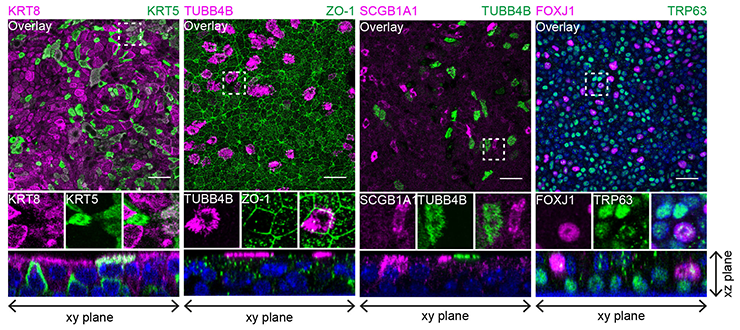
Co-staining of different epithelial markers on MTEC passage 2 after 8 days of air liquid interface (ALI) culture. From left to right, inserts were stained with basal cell marker KRT5 and luminal marker KRT8, cilia cell TUBB4B with tight junction protein ZO-1, secretory club cell marker SCGB1A1 with cilia marker TUBB4B and the last panel shows TRP63 positive basal cells with ciliated cell marker FOXJ1. Nuclei are stained with DAPI (blue). Scale bar, 30 µm. (Eenjes et al, 2018)
Moreover, it has been increasingly recognized that prenatal and perinatal events are major contributors to the susceptibility of chronic lung diseases. Not only intrinsic factors, like the genetic and epigenetic status of the lung cells, but also extrinsic factors, like smoke and infections, contribute to the development of an aberrant airway epithelium. The exact contributions of these factors are currently under investigation.
Vascular development
Pulmonary vascular diseases (PVD) encompass life-threatening clinical problems associated with several congenital or acquired anomalies, like congenital diaphragmatic hernia (CDH), Alveolar Capillary Dysplasia (ACD) and pulmonary hypertension of the newborn (PHN). Although the clinical symptoms may vary, the affected newborns all suffer from respiratory insufficiency with pulmonary hypertension as the major contributing factor. The unpredictable responses of patients towards current therapies still lead to a high morbidity and mortality. Treatment modalities are mostly non-evidence based and mainly rely on clinical expertise in the absence of randomized controlled trials with enough power. PVD share characteristic structural vascular abnormalities consisting of altered vascular development and changes in vascular remodeling. The pathology is characterized by increased muscularization of the arterioles and neo-muscularization of the capillaries, leading to progressive narrowing of the vascular lumen. We have shown that these structural abnormalities already exists prematurely in future CDH patients (Sluiter et al, 2013).
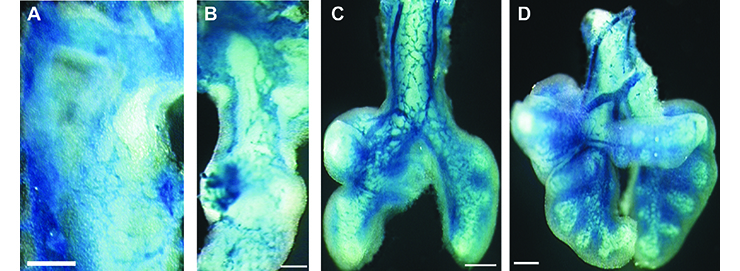
Early pulmonary vascular development (blue) visualized in the Tie2-LacZ transgenic mouse lung at E9.5 (A), E10.5 (B), E11.5 (C) and E12.5 (D). (Parera et al, 2005)
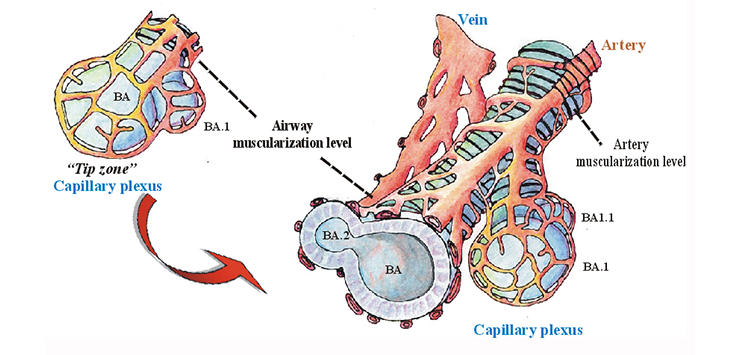
Distal angiogenesis as the model for pulmonary vascular development (Parera et al, 2005)
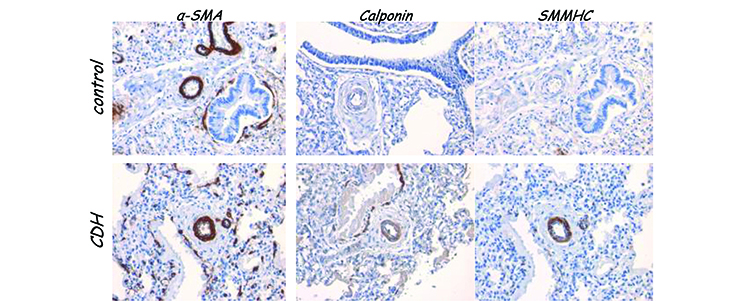
Normal and CDH human lungs at 30 weeks of gestation stained with the general smooth muscle cell marker -smooth muscle actin (left), or with two markers that detect more contractile markers (middle and right). This shows that premature CDH lungs already contains smooth muscle cells that express more contractile markers. (Ilona Sluiter)
We are interested in the pulmonary vascular development and the cells that are fundamental in the angiogenic process in relation to PVD. How and when do the structural abnormalities observed in PVD emerge? We have already shown that the pulmonary vasculature develops as an expansion of an existing vascular network that surrounds the primitive lung bud, indicating that the angiogenic process is active early in lung development. Moreover, our data showed that the perivascular cells, surrounding the expanding vessels, are affected in PVD. The pathways underlying the (ab)normal pulmonary response in case of pulmonary hypertension are evaluated a variety of techniques.
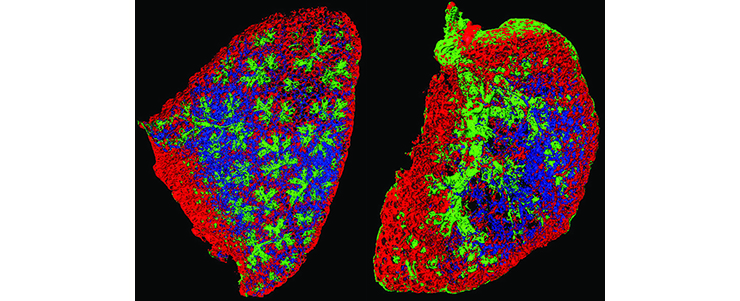
Embryonic complete left lobe of a mouse lung isolated at E15.5 of normal (left) and CDH (right) mice stained with antibodies to detect endothelial cells (blue), perivascular cells (red) and smooth muscle cells (green). The images clearly shows the extensive muscularization in the CDH lung, as well as a simplification of the lung structure, (Heleen Kool, Petra Burgisser)
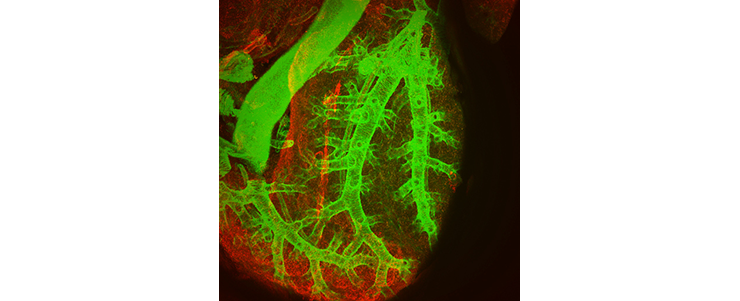
Whole mount immunostaining of a mouse CDH lung showing smooth muscle cells (green) and perivascular cells (red) (Heleen Kool, Petra Burgisser)
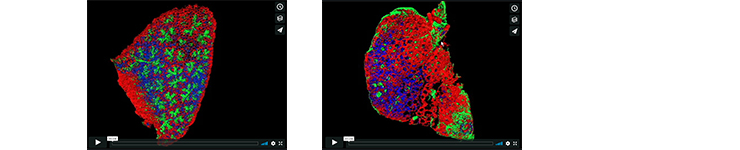
Whole mount immunostaining of the left lung of a control (left) or CDH (right) mouse at E15.
Vessels are blue (CD31), perivascular cells are red (NG2), and smooth muscle cells are green (α-Sma).
Diaphragm development
The defining deficiency in CDH is the diaphragm, which has varying degrees of defects. Through the hole in the diaphragm the abdominal organs are partially displaced into the thoracic cavity, leading to compromised lung growth and hypoplasia. Given the combined deficiency of the diaphragm defect and the pulmonary hypertension in CDH, we aim to identify common mechanisms that are active and maybe defective in the developing diaphragm and lung.
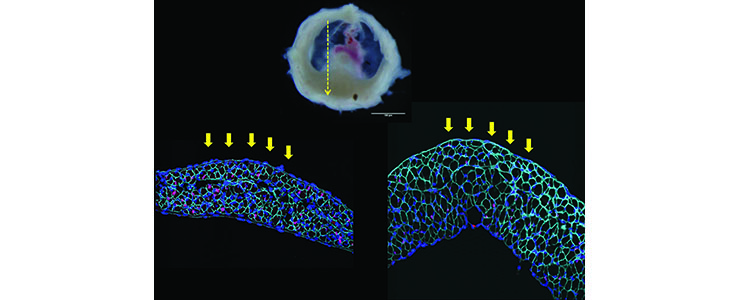
Staining of embryonic mouse diaphragms isolated at E16 .5 and E18.5 with antibodies to show satellite cells (red) and the basal lamina (purple). (Koji Nagata)
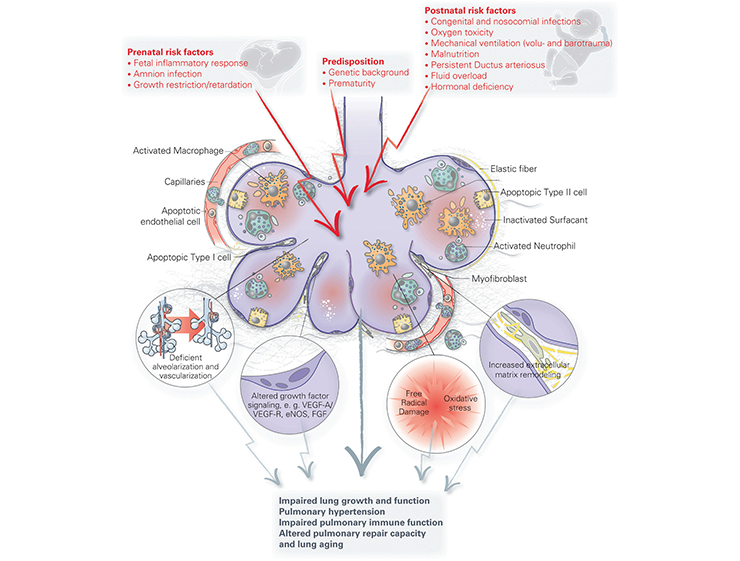
Bronchopulmonary dysplasia
Bronchopulmonary dysplasia (BPD) is a common adverse outcome affecting at least 25% of very preterm neonates (<32 weeks gestational age). These premature infants are born at the (early) saccular stage of lung development when respiratory ducts and saccules are just forming. These relative simple lungs are barely capable of supporting extra-uterine life, and therefore these children require intensive care. They are at risk of developing lung damage as a result of lung injury caused by ventilation, hyperoxia and inflammation, leading to an arrest in alveolar and microvascular development. Children with BPD more frequently develop respiratory infections, asthma - and emphysema-like symptoms later in life compared to term-born children. Understanding how the alveoli develop, repair and regenerate after injury is critical for the development of therapies, as currently there is no preventive strategy or cure for this chronic neonatal lung disease. We are interested in a specific population of resident progenitor cells, mesenchymal stromal cells, which are plastic cells with repair capacity. Additionally, we are interested in the role of the lung microbiome in perinatal lung development and the susceptibility of premature newborns exposed to infections or antibiotics to develop BPD.
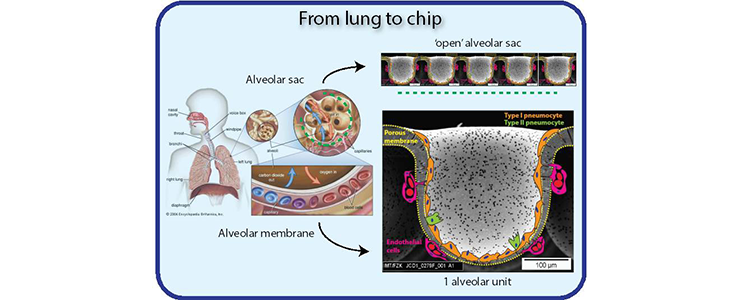
Lung on a Chip
In collaboration with Prof Hiemstra (LUMC), Prof Stamatialis and Dr Poot (U-Twente) and Dr Truckenmuller (MERLN, Maastricht), we are interested in developing an alveolar lung mimetic. Currently, two PhD students are focusing on the material science part (Danielle Baptista and Thijs Pasman), and one is dedicated to optimize the biological part (Sander van Riet). Collectively, we aim to provide a valuable tool for evaluating novel compounds and cell therapies for lung tissue regeneration. Furthermore, this model may be useful for future development of engineered alveolar tissue for implantation (for review see K. A. Schilders et al., Respir Res 17, 44: 2016).
Lung Models
In our research, we are using several approaches to investigate lung-specific mechanisms.
A short overview:
Embryonic lung explant
Mouse embryonic lungs can be isolated very early in development and grown at air-liquid interface. This allows the embryonic lungs to continue the early branching morphogenetic events.
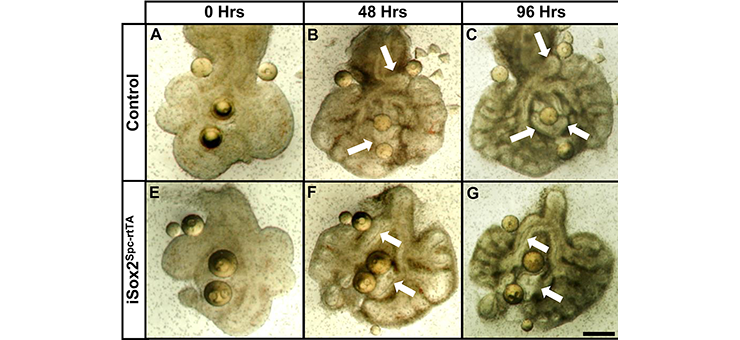
Lung explants of control (A–D) and iSox2Spc-rtTA (E–H) mice were grown for 96 hours in medium containing doxycycline. Heparin beads coated with Fgf10 were placed with the explants. The airways of control lungs that are in close proximity of the beads grow toward the Fgf10 source (arrows in B and C), whereas the iSox2SPC-rtTA derived lungs show severe reduction of this effect (arrows in F and G)
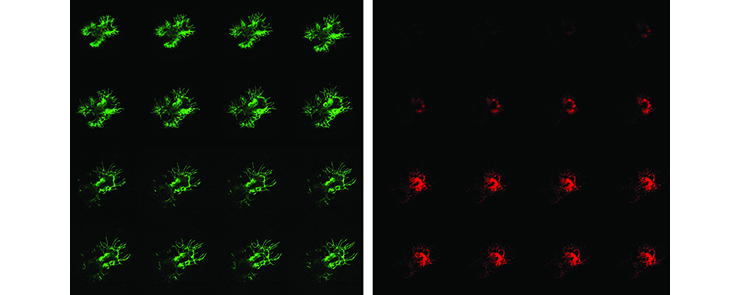
Time-lapse images of embryonic lung explant derived from VecCre * Rosa-YFP * Cspg4-dsRed transgenic mouse showing emerging vessels (green) and perivascular cells (red). (Heleen Kool and Petra Burgisser)
Endoderm-free epithelial cultures
Embryonic lungs are isolated and stripped of the surrounding mesenchyme. The mesenchyme-free endoderm tubes are cultured in matrigel with Fgf10.
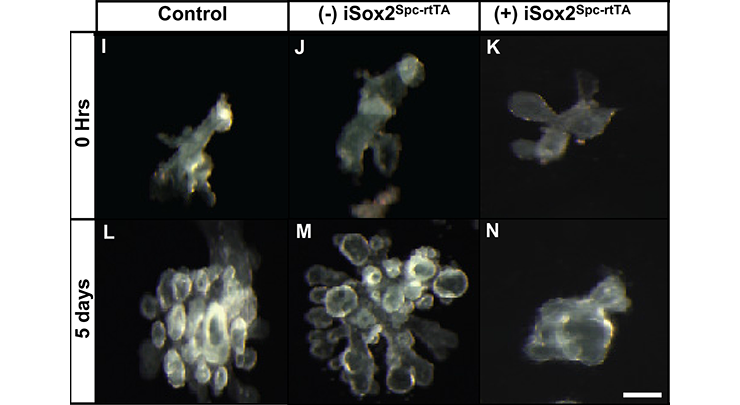
Mesenchyme-free lung endoderm from control and iSox2SPC-rtTA lungs was cultured in growth factor–reduced matrigel supplemented with Fgf10 in the presence (I, K, L, N) or absence (J, M) of doxycycline. The control endoderm (I, L) and the iSox2SPC-rtTA-derived endoderm grown in the absence (2) of doxycycline (J, M) clearly showed branching after 5 days of culture. iSox2SPC-rtTA-derived endoderm grown in the presence of doxycycline showed no branching (K, N).
Air Liquid Interface (ALI) cultures
Human or mouse airway epithelial cells are isolated and cultured at the air-liquid interface in a Transwell system. Upon exposure to air, these cultures differentiate into airways with differentiated ciliated -, mucous producing - and basal cells.
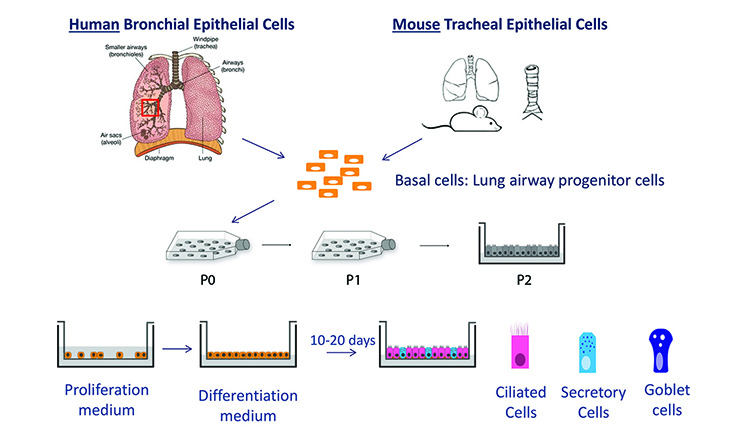
Graphic representation of the the ALI culture method (Evelien Eenjes; Eenjes et al, 2018)
Organoids
Human or mouse lung organoids are initiated from single lung cells and cultured to propagate and to investigate the differentiation potential of airway cells.
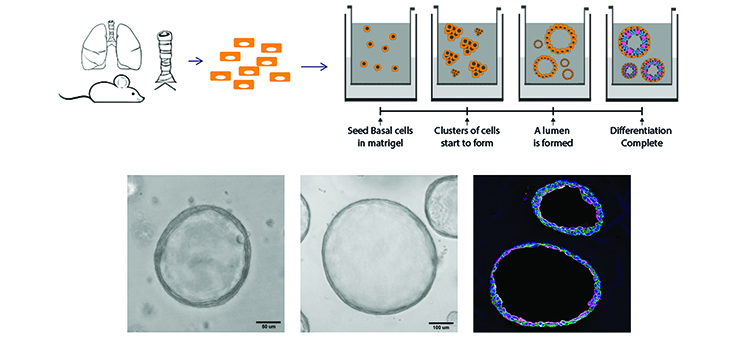
Representation of the lung organoids, including light microscopic images and IF image Krt5 (green) and Krt 8 (pink). (Evelien Eenjes, Lisette de Bruijn and Anneloes van Krimpen)
Mouse models
Various transgenic – and knockout mice are available in the laboratory to study the development of the pulmonary vasculature and the airways.
Education and career
Professional biography
June 1991 - December 1996: PhD student, faculty of Cell Biology and Genetics, Erasmus University, Rotterdam, The Netherlands
(1991 - 1993), and department of Genetics, St. Jude Children s Research Hospital, Memphis, TN, USA
(1993 - 1996). Title thesis: “Expression pattern of lysosomal protective protein/cathepsinA: Implications for the analysis of human galactosialidosis”, promotor: Prof dr. H. Galjaard, co-promotor: Dr. A. d’Azzo
January 1997 - December 1997: Research specialist at the department of Genetics, St. Jude Children s Research Hospital, Memphis,TN, USA.
February 1998 - December 2000: Post-doctoral fellow at the Department of Cell Biology, Erasmus MC, The Netherlands, in the laboratory of Prof dr S. Philipsen and Prof dr F.G. Grosveld (NWO fellowship).
January 2001 - December 2002: Senior investigator/group leader at the Department of Pediatric Surgery of the Sophia Children’s Research Hospital/Erasmus MC, The Netherlands, with Prof dr D. Tibboel.
January 2003 - November 2012: Assistant Professor and head of the laboratory at the Department of Pediatric Surgery of the Sophia Children’s Hospital/Erasmus MC, The Netherlands, with Prof dr D. Tibboel and Prof dr R. Wijnen.
From December 2012 - March 2022: Associate professor and head of the laboratory at the Department of Pediatric Surgery of the Sophia Children’s Hospital/Erasmus MC, The Netherlands, with Prof dr D. Tibboel and Prof dr R. Wijnen.
From April 2022: Full professor "Developmental biology of congenital pulmonary malformations" and head of the laboratory at the Department of Pediatric Surgery of the Sophia Children's Hospital/Erasmus MC, The Netherlands, with Prof. dr. R. Wijnen.
Official appointments:
- Editorial board member AJP-Lung
- NRS member Sweringa committee (2015-?)
- NRS Nationaal Programma Longonderzoek, Taskforces “Innovative Research” and “Cross fertilization between research areas”.
- ERS National delegate (2013-2016)
- ERS chair assembly 7.08 “Lung and airway developmental biology” (2017-?)
- ErasmusSophia Advies Commissie Research (2017-?)
Funding details:
Available on request.

Presentation at the ERS annual meeting in Milan 2017, Year in review
Publications
A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture. Evelien Eenjes, Tinne C. J. Mertens, Marjon J. Buscop-van Kempen, Yolanda van Wijck, Christian Taube, Robbert J. Rottier & Pieter S. Hiemstra. (2018). Sci Rep. 2018 May 9;8(1):7349.
Clinically relevant timing of antenatal sildenafil treatment reduces pulmonary vascular remodeling in congenital diaphragmatic hernia. (2016). Daphne S Mous, Heleen M Kool, Marjon J Buscop-van Kempen, Anton H Koning, Oleh Dzyubachyk, Rene M H Wijnen, Dick Tibboel, Robbert J Rottier. (2016). Am J Physiol Lung Cell Mol Physiol. 2016 Oct 1;311(4):L734-L742.
Regeneration of the lung: Lung stem cells and the development of lung mimicking devices. Kim A A Schilders, Evelien Eenjes, Sander van Riet, André A Poot, Dimitrios Stamatialis, Roman Truckenmüller, Pieter S Hiemstra, Robbert J Rottier. (2016). Respir Res. 2016 Apr 23;17:44.
Differentiated type II pneumocytes can be reprogrammed by ectopic Sox2 expression. Joshua Kapere Ochieng, Kim Schilders, Heleen Kool, Marjon Buscop-van Kempen, Anne Boerema-De Munck, Frank Grosveld, Rene Wijnen, Dick Tibboel, Robbert J Rottier. (2014). PLoS One. 2014 Sep 11;9(9):e107248.
Sox2 regulates the emergence of lung basal cells by directly activating the transcription of Trp63. Joshua K Ochieng, Kim Schilders, Heleen Kool, Anne Boerema-De Munck, Marjon Buscop-Van Kempen, Cristina Gontan, Ron Smits, Frank G Grosveld, Rene M H Wijnen, Dick Tibboel, Robbert J Rottier. (2014). Am J Respir Cell Mol Biol. 2014 Aug;51(2):311-22.
Exportin 4 mediates a novel nuclear import pathway for Sox family transcription factors. Cristina Gontan, Thomas Güttler, Erik Engelen, Jeroen Demmers, Maarten Fornerod, Frank G Grosveld, Dick Tibboel, Dirk Görlich, Raymond A Poot, Robbert J Rottier. (2009). J Cell Biol. 2009 Apr 6;185(1):27-34.
Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Cristina Gontan, Anne de Munck, Marcel Vermeij, Frank Grosveld, Dick Tibboel, Robbert Rottier. (2008). Dev Biol. 2008 May 1;317(1):296-309.
Distal angiogenesis: a new concept for lung vascular morphogenesis. Marta Canis Parera, Marieke van Dooren, Marjon van Kempen, Ronald de Krijger, Frank Grosveld, Dick Tibboel, Robbert Rottier. (2005). Am J Physiol Lung Cell Mol Physiol. 2005 Jan;288(1):L141-9.
A complete overview of publications can be found here:
Theses (co-promotor)
-
Prapapan Rajatapiti (June, 2007)
-
Cristina Gontan (April, 2008)
-
Ilona Sluiter (February, 2012)
-
Yadi Huang (June, 2012)
-
Lalini Raghoebir (Oktober, 2012)
-
Joshua Ochieng (June, 2014)
-
Kim Schilders (June, 2016)
-
Daphne Mous (November, 2017)
-
Heleen Kool (January, 2018)
-
Evelien Eenjes (April, 2020)
Scheduled theses:
-
Sander van Riet (2019)
-
Gabriela Edel (2021)
-
Evelien Slot (2021)
Student projects
Contact information and team members
Name: R. Rottier
Telephone: +31 10 704 41 40
E-mail
Visiting address
Dept. of Cell Biology
Erasmus MC
Faculty building, room Ee-1034B
Wytemaweg 80
3015 CN Rotterdam
The Netherlands
Mail address
Dept. of Cell Biology
Erasmus MC
Faculty building, room Ee-1034B
PO Box 2040
3000 CA Rotterdam
The Netherlands
Current people
 |
Prof. dr. R.M.H. Wijnen, head of department of Pediatric Surgery |
 |
Isabel Sreeram, PhD student |
 |
Prof. dr. D. Tibboel, |
 |
Cinta Iriondo, PhD student |
 |
Gabriela Edel, |
 |
Marjon Buscop-van Kempen, technician |
 |
Floor Benthem, |
 |
Anne Boerema-de Munck, technician |
|
Sem Koornneef, PhD student |
 |
Dr. Marco Schnater, |
Alumni
(in alphabetical order):
- Beurskens, Niels (PhD Student)
- Bhalla, Savita (scientist)
- Burgisser, Petra (technician)
- Collins,Jennifer (postdoctoral fellow)
- Eenjes, Evelien (PhD student)
- Felix, Janine (PhD Student)
- Gontan, Cristina (PhD Student)
- Horst, Irene van der (PhD Student)
- Huang, Yadi (PhD Student)
- Kool, Heleen (PhD Student)
- Lohman, Frans (post-doc)
- Meyboom, Joël (technician)
- Mous, Daphne (PhD student)
- Nagata, Koji (visiting pediatric surgeon)
- Ochieng, Joshua Kapere (PhD Student)
- Parera, Marta Canis (PhD Student)
- Raghoebir, Lalini (PhD Student)
- Rajatapiti, Prapapan (PhD student)
- Schilders, Kim (PhD Student)
- Sluiter, Ilona (PhD Student)
- Snoeren, Sylvia (technician)
- Ven, Kees van der (Pediatric surgeon)


