About Pines-Alex
Introduction

Alex Pines received his PhD in Biochemistry in 2005 from the University of Trieste, Italy. He is a cell and molecular biologist with 15+ years’ experience in DNA damage repair pathways, genome stability and age-related diseases. He is a member of the Department of Molecular Genetics since 2015. His research focuses on elucidating the repair mechanisms of transcription interfering lesions and associated diseases.
"We apply a multi-disciplinary approach that involves proteomics, live imaging, and functional genomics, with a particular interest in transcription-associated repair processes."
Research topic

The goal of Pines’ research is to reveal the molecular details of DDR to fully comprehend the root causes of TILs driving progressive neurodegeneration. Recent advances in contemporary experimental approaches, such as sophisticated live cell imaging, CRISPR-mediated genome editing, and genome-wide omics have set the scene to take up this challenging research objectives:
- Genome-wide DNA repair distribution
- Composition and dynamics of DNA repair complexes during the different stages of the repair process
- Cell type-specific responses to TILs
- Determine pathophysiological consequences
Field(s) of expertise
DNA Damage Response
Persistent DNA damage and the consequent transcription stress disturb cellular homeostasis. TILs and accompanying transcription stress trigger a strong DNA Damage Response (DDR) that coordinates key cellular processes, mainly via dynamic post-translational protein modifications (PTMs). These PTM- mediated DDR events are thought to target DNA repair, transcription, pre-mRNA splicing and export, chromatin, the translation and protein quality control machineries.
Insight into TIL-induced DDR-associated PTMs changes, and their physiological impact, thus, provide a window to define the fundamental causes of the major age-related neurologic diseases and to develop interventions that alleviate the root cause of ageing-associated morbidity.
Transcription-Coupled Nucleotide Excision Repair
Transcription-Coupled Nucleotide Excision Repair (TC-NER) represents the major caretaker of the genome to preserve the transcription programs required for normal cellular functioning and is the focus of our research. TC-NER is activated by binding of its critical factors CSA, CSB and UVSSA to stalled RNAPII. CSB is a DNA-dependent ATPase, its translocating activity is essential for recruiting CSA, which is the substrate-adaptor of the Culin4-RING ubiquitin E3 ligase complex (CRL4CSA), and UVSSA, that brings in the broad-spectrum de-ubiquitylation enzyme USP7. An intricate dynamic molecular interplay between CSA-, CSB- and UVSSA containing complexes is required to coordinate the removal of DNA lesion and subsequent transcriptional restart.
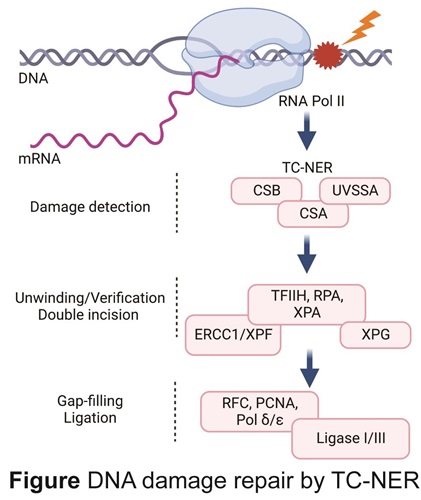
Following TC-NER factor binding, UVSSA recruits the basal transcription factor TFIIH and XPA to verify the lesion. Subsequently, the single-stranded DNA-binding complex RPA orients the 5’ and 3’ structure-specific endonucleases ERCC1-XPF and XPG to excise the damaged strand, followed by gap filling DNA synthesis and DNA ligation.
Inherited TC-NER-deficiency is associated with serious health threats caused by persistence of RNAPII stalled at endogenously DNA damage, which gives rise to Cockayne syndrome (CS), characterized by cachexia, severe progressive neurological decline and accelerated aging.
Publications

Selected publications
- N. Kumar, et al. “Global and transcription-coupled repair of 8-oxoG is initiated by nucleotide excision repair proteins.” Nature communication 2022
- F Liebelt, et al. “Transcription-coupled nucleotide excision repair is coordinated by ubiquitin and SUMO in response to ultraviolet irradiation” Nucleic Acids Res. 2020
- F Wienholz, et al. “FACT subunit Spt16 controls UVSSA recruitment to lesion-stalled RNA Pol II and stimulates TC-NER” Nucleic Acids Res. 2019
- A Pines, et al “TRiC controls transcription resumption after UV damage by regulating Cockayne Syndrome protein A” Nature communication 2018
- MS Luijsterburg, et al. “PARP1 Links CHD2-Mediated Chromatin Expansion and H3.3 Deposition to DNA Repair by Non-homologous End-Joining” Mol Cell. 2016
- A Pines, et al. “Touching base with PARPs: Moonlighting in the repair of UV lesions and double-strand breaks.” Trends in Biochemical Sciences 2013
- A Pines, et al. “PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1.” The Journal of Cell Biology 2012
- A Pines, et al. “Global phosphoproteome profiling reveals unanticipated networks responsive to cisplatin treatment of embryonic stem cells.” Molecular and cellular biology 2011
Teaching activities
Alex Pines teaches in the Nanobiology BSc program of Erasmus University and Delft University, where he is course manager and teacher of Journal Club2.

Research Topic
Alex Pines has built up his own, independent, line of research embedded into Prof Wim Vermeulen’s laboratory . As part of the Molecular Genetics department, with its central research focus on the
"DNA damage response",Alex Pines’ group closely collaborates with several PIs of the department and with Dr. Arjan F. Theil, consultant scientist of the expertise center ENCORE at ERASMUS MC. Alex Pines has established several national and international collaborations in the DNA repair field, guaranteeing an excellent international network and a scientific embedment of his research.
Lately, he has joined forces with Dr. Willianne I.M. Vonk and Dr. Wilbert Vermeij (Genome Instability & Nutrition, Princess Máxima Center (PMC) to study DNA damage-associated protein quality control in neurodegeneration.




